


Khan Academy Avogadro's Number
Home > Chemistry B - Course OutlineStudents may access the online textbook by following this link. The Access Code is E8D52F60E7. I have found that the online textbook does not currently work well with Google Chrome. The study guide is access by clicking on the chapter titles. Here is a Blank Lesson Note Page for your video notes. | Unit/Chapter | Websites and Videos | Chapter 7
| Ionic Bonding (Brightstorm)
Properties of Ionic Compounds (Brightstorm)
Ionic and Covalent Bonding (Khan Academy)
Crystal Structures (Khan Academy)
Metallic Bonding (Brightstorm) | Chapter 8
| Covalent Bonding (Brightstorm)
Lewis Electron Dot Structures (Brightstorm)
Bond Polarity (Brightstorm)
Intermolecular Forces (Khan Academy)
Intermolecular Forces (Brightstorm) | Chapter 9
| Basic Chemical Equations (Brightstorm)
Balancing Chemical Equations (Khan Academy)
Balancing Chemical Equations (Brightstorm)
Types of Reactions (Brightstorm)
Synthesis (or Direct Combonation) (Brightstorm)
Decomposition (Brightstorm)
Combustion (Brightstorm)
Single Replacement (Brightstorm)
Double Replacement (Brightstorm) | Chapter 10
| Mole Calculations with Avogadro's Number (Brightstorm)
The Mole and Avogadro's Number (Khan Academy)
Mole Calculations with Molar Mass (Brightstorm)
Empirical and Molecular Formulas (Brightstorm)
Empirical and Molecular Formulas (Khan Academy)
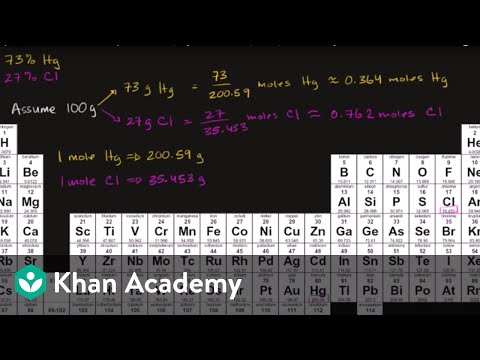
% Composition to Empirical Formulas (Khan Academy)
Empirical Formulas Sample Problem (Khan Academy)
Hydrates (Brightstorm) | Chapter 11
| Stoichiometry (Khan Academy)
Basic Stoichiometry (Brightstorm)
Stoichiometry Example Problem 1 (Khan Academy)
Stoichiometry Example Problem 2 (Khan Academy)
Limiting Reactants (Khan Academy)
Limiting Reactants (Brightstorm)
Limiting Reactant Example Problem (Khan Academy)
Percent Yield (Brightstorm) | Chapter 14
| Solution Terminology (Khan Academy)
Types of Solutions (Brightstorm)
Solvation (process of dissolving) (Brightstorm)
Solubility (Khan Academy)
Molarity vs. Molality (Brightstorm)
Colligative Properties (Khan Academy)
Colligative Properties (Brightstorm)
Vapor Pressure Lowering (Brightstorm)
Boiling Point Elevation (Brightstorm)
Freezing Point Reduction (Brightstorm) | Chapter 18
| Introduction to Acids and Bases (Khan Academy)
Properties of Acids and Bases (Brightstorm)
Neutralization-Hydrolysis Reactions and Salts (Brightstorm)
Bronstead-Lowry Model for Acids and Bases (Brightstorm)
Conjugate Acids and Bases (Khan Academy)
Conjugate Acids and Bases (Brightstorm)
Acids/Bases Equilibrium (Brightstorm)
Strong Acids and Bases; pH and pOH (Khan Academy)
pH and pOH (Brightstorm)
Ka - Weak Acids (Khan Academy)
Kb - Weak Bases (Khan Academy)
|
|
|
Khan Academy Avogadro's Numbers
The Mole, Avogadro’s Number, and Counting by Mass (or Weight!) This video introduces counting by mass, the mole, and how it relates to atomic mass units (AMU) and Avogadro’s number. The Mole and Avogadro’s Number. Introduction to the idea of a mole as a number (vs. A video demonstration of The Mole and Avogadro's Number from Salman Khan of Khan Academy. Concentrations of Solutions Calculations Brief explanations of terms and formula plus variations of the formula. Dilution Calculations and examples A clear description and example of creating dilutions. Chemistry library - Atoms, compounds, and ions - Introduction to the atom - The mole and Avogadro's number. Plus there's a whole new unit, More about atoms, which contains two playlists, each with just one module that has the same name as the playlist: Moles and molar mass - Moles and molar mass - This module is an exercise. The number 6.022 × 10²³ is known as Avogadro's number. The Mole and Molar Mass, Study Guide 📖 This study guide explains the concept of a mole, and provides examples of calculating the molar mass of: Al(NO 3 ) 3, Ba(SCN) 2, CO, N 2, Ar, HCl, CaSO 4 ⋅ 1 ⁄ 2 H 2 O, Ca(C 2 H 3 O 2 ) 2, (HOOCCH 2 ) 2 NCH 2 CH 2 N(CH 2 COOH) 2,.




